Novel polymeric derivatives of betulin with anticancer activity†
Abstract
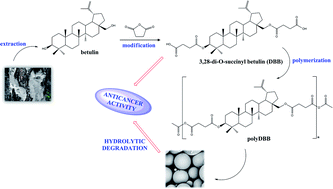
In order to provide novel polymeric biomaterials for chemotherapeutic purposes, in this paper we described the synthesis and the characterization of the physicochemical properties of a betulin-based polyanhydride exhibiting anti-cancer effects. The polyanhydride was obtained by a melt polycondensation of a disuccinate betulin (3,28-di-O-succinyl betulin), and was thoroughly characterized through 1H NMR and 13C NMR spectroscopies, correlation spectroscopy, heteronuclear single quantum correlation, size exclusion chromatography, differential scanning calorimetry and FT-IR spectroscopy. It was confirmed, that the obtained polyanhydride undergoes hydrolytic degradation, releasing disuccinate betulin as a degradation product. Polyanhydride of a disuccinate betulin was tested for cytostatic activity against a wide range of cancer cell lines (HeLa, MCF-7, A-549, U-87MG, KB and HepG2), proving its efficiency in inhibiting the growth of selected cancer cells. To realize the concept of an easily administrated drug release system, polyanhydride was fabricated in a form of micro- (1–30 μm) and nanospheres (∼400 nm) by using an emulsion solvent evaporation method. The micro- and nanospheres were characterized by SEM.


 Please wait while we load your content...
Please wait while we load your content...