Environmental surface chemistries and adsorption behaviors of metal cations (Fe3+, Fe2+, Ca2+ and Zn2+) on manganese dioxide-modified green biochar†
Abstract
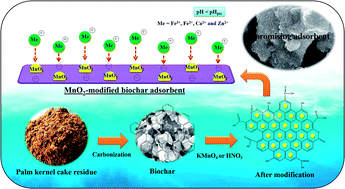
The facile preparation and modification of low-cost/efficient adsorbents or biochar (CP) derived from the carbonization of palm kernel cake (lignocellulosic residue) has been studied for the selective adsorption of various metal cations, such as Fe3+, Fe2+, Ca2+ and Zn2+, from aqueous solution. The CP surface was modified with KMnO4 (CPMn) and HNO3 (CPHNO3) in order to improve the adsorption efficiency. The physicochemical properties of the as-prepared adsorbents were investigated via BET, pHpzc, FT-IR, Boehm titration, TG-DTG, XRD and SEM-EDS techniques. The surfaces of all adsorbents clearly demonstrated negative charge (pHpzc > pH of the mixture solution), resulting in a high adsorption capacity for each metal cation. Fe2+ was found to be more easily adsorbed on modified CP than the other kinds of metal cations. Synergistic effects between the carboxylic groups and MnO2 on the surface of CPMn resulted in better performance for metal cation adsorption than was shown by CPHNO3. The maximum adsorption capacities for Fe3+, Fe2+, Ca2+ and Zn2+ using CPMn, which were obtained from a monolayer adsorption process via Langmuir isotherms (R2 > 0.99), were 70.67, 68.60, 5.06 and 22.38 mg g−1, respectively. The adsorption behavior and monolayer-physisorption behavior, via a rapid adsorption process as well as single-step intra-particle diffusion, were also verified and supported using Dubinin–Radushkevich, Redlich–Peterson and Toth isotherms, a pseudo-second-order kinetic model and the Weber–Morris model. Moreover, the thermodynamic results indicated that the adsorption process of metal cations onto the CPMn surface was endothermic and spontaneous in nature. This research is expected to provide a green way for the production of low-cost/efficient adsorbents and to help gain an understanding of the adsorption behavior/process for the selective removal of metal ions from wastewater pollution.


 Please wait while we load your content...
Please wait while we load your content...