Syntheses, structures, and magnetic properties of mixed-ligand complexes based on 3,6-bis(benzimidazol-1-yl)pyridazine†
Abstract
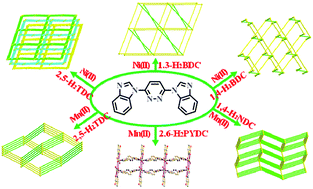
Six new metal–organic coordination polymers (CPs) [Ni(L)(2,5-TDC)(H2O)]n (1), [Ni(L)(1,3-BDC)(H2O)]n (2), [Ni(L)(1,4-BDC)(H2O)]n (3), [Mn(L)(2,5-TDC)(H2O)]n (4), [Mn(L)(2,6-PYDC)(H2O)]n (5) and [Mn(L)(1,4-NDC)]n (6) were achieved by reactions of the corresponding metal salt with mixed organic ligands (L = 3,6-bis(benzimidazol-1-yl)pyridazine, 2,5-H2TDC = thiophene-2,5-dicarboxylic acid, 1,3-H2BDC = isophthalic acid, 1,4-H2BDC = terephthalic acid, 2,6-H2PYDC = pyridine-2,6-dicarboxylic acid, 1,4-H2NDC = naphthalene-1,4-dicarboxylic acid) under solvothermal condition. CPs 1–6 were characterized by single-crystal X-ray diffraction, IR, TG, XRD and elemental analyses. Their structures range from the intricate 3D CPs 1, 3, 4 and 6 to the 2D coordination polymer 2 and the infinite 1D chain 5. The CPs 1–4 and 6 underlying networks were classified from the topological viewpoint, disclosing the distinct sql (in 1), pcu (in 3 and 6), new topology (in 2), and dia (in 4) topological nets. Moreover, analysis of thermal stability shows that they had good thermal stability. Finally, magnetic properties of CPs 1–6 have been studied, the results showed that complex 2 had ferromagnetic coupling and complexes 1, 3–6 were antiferromagnetic.


 Please wait while we load your content...
Please wait while we load your content...