Armchair shaped polymeric nitrogen N8 chains confined in h-BN matrix at ambient conditions: stability and vibration analysis†
Abstract
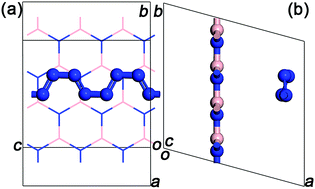
A new hybrid material comprising of armchair shaped polymeric nitrogen chains (N8) encapsulated in h-BN matrix is proposed and studied through ab initio calculations. Interestingly, the theoretical results demonstrate that N8 chains, confined in h-BN matrix, are effectively stabilized at ambient pressure and room temperature. Moreover, N8 chains can dissociate and release energy at a much milder temperature of 600 K. The confined polymer N8 unit needs to absorb 0.68 eV energy to span the decomposition energy barrier before decomposing. Further research shows that the charge transfer between N8 chain and h-BN layer is the stabilizing mechanism of this new hybrid material. And the low dissociation temperature is due to a much smaller amount of charge transfer compared to other confined systems in previous reports. The IR and Raman vibrational analyses suggest that host–guest interactions in the hybrid material influence the vibration modes of both the confined N8 chain and h-BN layer.


 Please wait while we load your content...
Please wait while we load your content...