Supramolecular gels from sugar-linked triazole amphiphiles for drug entrapment and release for topical application†
Abstract
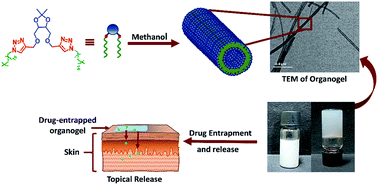
A simple molecular framework obtained by cross-linking a hydrophobic chain with S,S- and R,R-tetritol by the copper-catalysed azide–alkyne cycloaddition reaction is found to serve as an excellent bioisostere for self-assembly. The hexadecyl-linked triazolyl tetritol composite spontaneously self-assembles in n-hepane and methanol to form hierarchical organogels. Microscopic analyses and X-ray diffraction studies demonstrate eventual formation of nanotubes through lamellar assembly of the amphiphiles. A rheological investigation shows solvent-dictated mechanical properties that obey power law behavior similar to other low molecular weight gelators (LMOGs). The gel network was then utilized for the entrapment of drugs e.g. ibuprofen and 5-fluorouracil, with tunable mechanical behaviour under applied stress. The differential release profiles of the drugs over a period of a few hours as a result of the relative spatio-temporal location in the supramolecular network can be utilized for topical formulations.


 Please wait while we load your content...
Please wait while we load your content...