Synthesis of tetracyclic indolin-3-ones through Pd-catalyzed intramolecular deacetylative dearomatization of 3-acetoxy-indoles†
Abstract
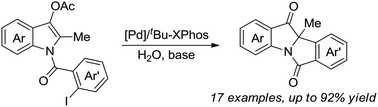
An efficient palladium-catalyzed intramolecular deacetylative dearomatization reaction of 3-acetoxyindoles has been developed. A range of tetracyclic indolin-3-ones bearing C2-quaternary stereocenters are achieved in good yields, showing a wide substrate scope for this reaction. A preliminary enantioselective reaction is established to furnish the product in 63% ee by using (R,R,R)-phosphoramide-PE as a chiral ligand.


 Please wait while we load your content...
Please wait while we load your content...