A fluorescence sensing platform of theophylline based on the interaction of RNA aptamer with graphene oxide†
Abstract
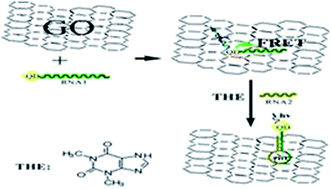
RNA, with a structure similar to DNA, should exhibit similar behaviors when it interacts with graphene. In this work, we designed a sensing platform of theophylline based on the interaction of an RNA aptamer with graphene oxide (GO) using the fluorescence as a sensing signal. Firstly, quantum dots (QDs) were modified with the selected ssRNA that can be used as an aptamer to recognize the theophylline. The fluorescence of QDs will be quenched in the presence of GO due to the noncovalent assembly between ssRNA aptamer and GO, leading to fluorescence resonance energy transfer (FRET) from QDs to GO, fluorescence “turn-off”. Then, in the presence of theophylline, the ssRNA aptamer recognizes theophylline to form a dsRNA–theophylline complex. The weak affinity between the complex and GO makes QDs move away from the GO surface, leading to the fluorescence recovery of QDs, fluorescence “turn-on”. Because of the high fluorescence quenching efficiency, unique structure of GO and specificity of the RNA aptamer, the proposed sensing platform exhibits high sensitivity and excellent selectivity for the determination of theophylline. The excellent performance of the sensor based on GO provides new opportunities for sensitive and selective detection of biorecognition events.


 Please wait while we load your content...
Please wait while we load your content...