Novel asymmetric photodimerization reaction of coumarin derivatives bearing a chiral 2-oxazolidinone auxiliary†
Abstract
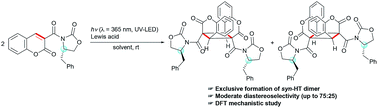
A novel asymmetric photodimerization reaction of coumarin derivatives bearing the (S)-4-benzyl-2-oxazolidinone auxiliary provides only the syn-head-to-tail (syn-HT) dimer with moderate diastereoselectivity (up to 75 : 25). The mechanism of complete syn-HT selectivity and moderate diastereoselectivity is proposed based on the result of density functional theory (DFT) calculation. The benzyl group of the (S)-4-benzyl-2-oxazolidinone auxiliary in combination with a Lewis acid exerts effective diastereofacial shielding of the reaction site.


 Please wait while we load your content...
Please wait while we load your content...