Discovery of methylpyrimidine ring-fused diterpenoid analogs as a novel testosterone synthesis promoter†
Abstract
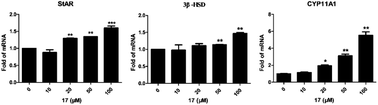
Herein we screened our small synthetic library of diterpenoid analogs for hit compounds on promoting testosterone synthesis and the methylpyrimidine ring-fused diterpenoid analog 7 was obtained as the hit. Based on the hit, a series of derivatives were designed, synthesized and evaluated for their effects on testosterone secretion in mouse Leydig TM3 cells. Most of the derivatives showed better activity in promoting testosterone synthesis than the positive control compound icariin, among which compound 17 has optimal activity and little cytotoxicity. Preliminary mechanism studies indicated that 17 significantly promoted the expression of testosterone synthesis-related marker genes (StAR, 3β-HSD and CYP11A1). Further studies showed that 17 provided sufficient steroid materials for testosterone synthesis by stimulating autophagy in Leydig cells. Thus compound 17 emerged as a potential lead compound for further development of therapeutics for late onset of hypogonadism (LOH).


 Please wait while we load your content...
Please wait while we load your content...