Controlled phase evolution from Cu0.33Co0.67S2 to Cu3Co6S8 hexagonal nanosheets as oxygen evolution reaction catalysts†
Abstract
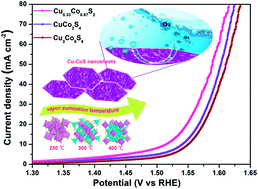
Developing cheap and efficient transition metal-based catalysts for the oxygen evolution reaction (OER) plays the key role in large-scale implementation of hydrogen production. However, there is still a lack of effective ways to tune the catalysts performance for the OER reaction from the aspect of structure design and element modulation simultaneously. Herein, a novel Cu0.33Co0.67S2 hexagonal nanosheet has been synthesized through the coprecipitation reaction followed by subsequent vapor sulfidation. Simply mixed with carbon nanotubes (CNTs) during electrode preparation, this Cu0.33Co0.67S2 exhibits an overpotential of 284 mV vs. RHE at a current density of 10 mA cm−2 in 1.0 M KOH. The improved OER performance of the Cu0.33Co0.67S2 electrode can be attributed to the electrocatalytically active sites involved in octahedral coordination structures and further activated by Cu substitution. The encouraging results provide insight into further rational design of transition metal-based electrochemical catalysts towards OER applications.


 Please wait while we load your content...
Please wait while we load your content...