Superparamagnetic nanoparticle-catalyzed coupling of 2-amino pyridines/pyrimidines with trans-chalcones†
Abstract
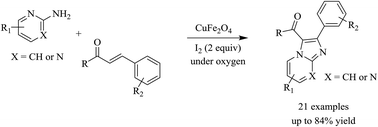
An aerobic coupling of 2-aminopyrimidines or 2-aminopyridines with trans-chalcones to afford aroylimidazo[1,2-a]pyrimidines and aroylimidazo[1,2-a]pyridines is reported. Reactions proceed in the presence of CuFe2O4 superparamagnetic nanoparticle catalyst, two equivalents of iodine, oxygen oxidant, and 1,4-dioxane solvent. The catalyst is superior to many common copper or iron complexes. Copper ferrite could be easily separated by magnetic decantation and reused up to 5 times without a major loss of activity. The method described here marks a rare example of using a simple, heterogeneous catalyst for synthesis of fused heterocycles. To our best knowledge, aroylimidazo[1,2-a]pyrimidines and aroylimidazo[1,2-a]pyridines were not previously synthesized using this protocol.


 Please wait while we load your content...
Please wait while we load your content...