Role of polyvinylpyrrolidone in the electrochemical performance of Li2MnO3 cathode for lithium-ion batteries†
Abstract
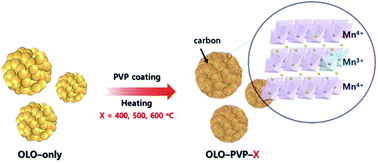
While Li2MnO3 as an over-lithiated layered oxide (OLO) shows a significantly high reversible capacity of 250 mA h g−1 in lithium-ion batteries (LIBs), it has critical issues of poor cycling performance and deteriorated high rate performance. In this study, modified OLO cathode materials for improved LIB performance were obtained by heating the as-prepared OLO at different temperatures (400, 500, and 600 °C) in the presence of polyvinylpyrrolidone (PVP) under an N2 atmosphere. Compared to the as-prepared OLO, the OLO sample heated at 500 °C with PVP exhibited a high initial discharge capacity of 206 mA h g−1 and high rate capability of 111 mA h g−1 at 100 mA g−1. The superior performance of the OLO sample heated at 500 °C with PVP is attributed to an improved electronic conductivity and Li+ ionic motion, resulting from the formation of the graphitic carbon structure and increased Mn3+ ratio during the decomposition of PVP.


 Please wait while we load your content...
Please wait while we load your content...