Strategies for the synthesis of fluorinated polyesters†
Abstract
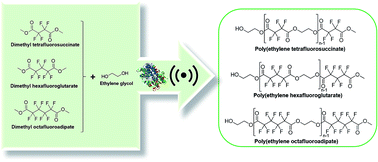
In this work we synthetized three fluorinated polyesters from dimethyl tetrafluorosuccinate (DMTFS), dimethyl hexafluoroglutarate (DMHFG), and dimethyl octafluoroadipate (DMOFA) and ethylene glycol. The influence of parameters like monomer's size, temperature, vacuum, ultrasound and catalyst, on the polyesters synthesis was evaluated. The conversion rates were assessed considering 1H NMR data and the results disclose the role of ultrasound (US) as crucial to attain high reaction conversion rates (≈20% of increase relatively to the reactions performed in absence of US). The effect of US was more relevant for the higher molecular weight monomers (DMHFG and DMOFA). The use of Candida antarctica lipase (immobilized CALB) marginally favors the synthesis reactions when fixing the other conditions. The size of the starting monomers influenced greatly the reaction conversion rates, as shorter monomers gave rise to high amount of product recovering. All the produced polyesters were isolated and fully characterized by NMR (1H and 19F), FTIR, TGA and MALDI-TOF.


 Please wait while we load your content...
Please wait while we load your content...