Experimental and theoretical evaluation on the antioxidant activity of a copper(ii) complex based on lidocaine and ibuprofen amide-phenanthroline agents†
Abstract
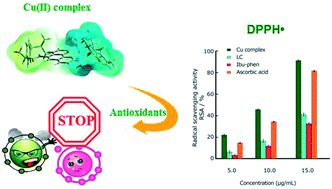
A new copper(II) complex, [Cu(LC)(Ibu-phen)(H2O)2](ClO4)2 (LC: lidocaine, Ibu-phen: ibuprofen amide-phenanthroline), was synthesized and characterized. The antioxidant activities of the free ligands and the copper(II) complex were evaluated by in vitro experiments and theoretical calculations using density functional theory (DFT). Structures of the ligand Ibu-phen and the complex were identified by 1H and 13C NMR, FT-IR spectroscopies, mass spectrometry, thermogravimetric analysis and elemental analysis. The antioxidant potentials of LC and Ibu-phen ligands as well as copper(II) complex were also evaluated by DPPH˙, ABTS˙+, HO˙ essays and EPR spectroscopy. The experimental results show that the radical scavenging activity (RSA) at various concentrations is decreased in the following order: copper(II) complex > ascorbic acid > LC > Ibu-phen. Structural and electronic properties of the studied compounds were also analyzed by DFT approach at the M05-2X/6-311++g(2df,2p)//M05-2X/LanL2DZ level of theory. ESP maps and NPA charge distributions show that the highly negative charge regions found on the N and O heteroatoms make these sites more favorable to bind with the central copper ion. Frontier orbital distributions of copper(II) complex indicate that HOMOs are mainly localized at Ibu-phen, while its LUMOs are distributed at LC. Based on natural bond orbitals (NBO) analyses, Cu(II) ion plays as electron acceptor in binding with the two ligands and two water molecules. Thermochemical properties including bond dissociation enthalpy (BDE), ionization energy (IE), electron affinity (EA), proton affinity (PA) characterizing three common antioxidant mechanisms i.e. hydrogen transfer (HT), single electron transfer (SET) and proton loss (PL) were finally calculated in the gas phase and water solvent for two ligands and the copper(II) complex at the same level of theory. As a result, the higher EA and lower BDE and PA values obtained for copper(II) complex show that the complex shows higher antioxidant potential than the free ligands.


 Please wait while we load your content...
Please wait while we load your content...