Molecular dynamics simulation of four typical surfactants in aqueous solution†
Abstract
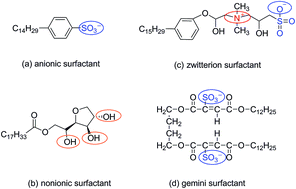
The thermodynamic values of the four surfactants, anionic surfactants, nonionic surfactants, zwitterion surfactants and gemini surfactants, were calculated by molecular dynamics simulation. The calculated results of thermodynamic parameters showed that the four surfactant can form micelles spontaneously. The mainly force for micellization process is entropy-driven, and as the temperature increases, the entropy-driven contribution is gradually reduced. There are linear enthalpy–entropy compensation phenomena for the four surfactants. Among the studied four surfactants, the gemini surfactant is the easiest to form micelles and has good stability, the zwitterion surfactant is the second, and the anionic surfactant is the least stable.


 Please wait while we load your content...
Please wait while we load your content...