Colloidal CdxZn1−xS nanocrystals as efficient photocatalysts for H2 production under visible-light irradiation†
Abstract
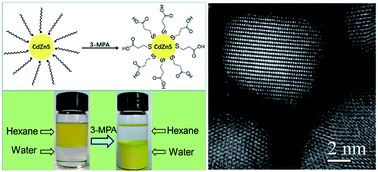
CdxZn1−xS nanocrystals with sizes ranging from 3–11 nm were synthesized by a simple organic solution method. The nanocrystals possess a cubic zinc-blende structure and the bandgap blue-shifts from 2.1 eV to 3.4 eV by increasing the composition of Zn ions in the solid solutions. After a facile ligand exchange process, the photocatalytic activity for H2 production of the CdxZn1−xS nanocrystals was investigated under visible-light irradiation (λ ≥ 420 nm) with Na2SO3/Na2S as the electron donor. It was found that the Cd0.8Zn0.2S had the highest photoactivity with H2 evolution rate of 6.32 mmol g−1 h−1. By in situ adding Pt precursors into the reaction solution, inhomogenous Pt–CdxZn1−xS nanoheterostructures were formed, which accounted for a 30% enhancement for the H2 evolution rate comparing with that of pure Cd0.8Zn0.2S nanocrystals. This work highlights the use of facile organic synthesis in combination with suitable surface modification to enhance the activity of the photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...