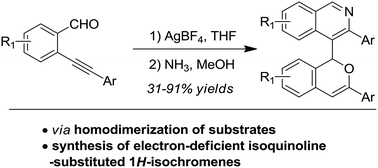
Synthesis of 4-(1H-isochromen-1-yl)isoquinolines through the silver-catalysed homodimerization of ortho-alkynylarylaldehydes and subsequent condensation of the 1,5-dicarbonyl motif with NH3†
Abstract
4-(1H-Isochromen-1-yl)isoquinoline derivatives were synthesized in high yields via the AgBF4-catalyzed self-reaction of ortho-alkynylarylaldehydes to give isochromene intermediates, followed by the dehydration of the 1,5-dicarbonyl motif with NH3. Compared with electron-rich aromatic substituents, this strategy can provide the desired isochromene products with an electron-deficient isoquinoline unit. The reactions feature simple experimental operations, mild reaction conditions and high product yields.


 Please wait while we load your content...
Please wait while we load your content...