Homogeneous hydroformylation of long chain alkenes catalyzed by water soluble phosphine rhodium complex in CH3OH and efficient catalyst cycling
Abstract
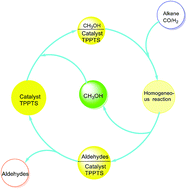
The hydroformylation of long chain alkenes catalyzed by a water soluble Rh/TPPTS complex (TPPTS: sodium salt of sulfonated triphenylphosphine) in methanol was investigated. The mixture of rhodium precursor HRh(CO)(TPPTS)3, ligand TPPTS, methanol and a long chain alkene becomes a single phase under reaction conditions, which make the hydroformylation reaction proceed homogeneously. Both the conversion of long chain alkene and the selectivity to aldehydes (including the aldehydes forming methylacetals) could reach up to 97.8% and 97.6%, respectively, with 3323 h−1 of TOF (TOF: turnover frequency is defined as the moles of converted alkene per mole of Rh per hour). After the solvent methanol was removed under the reaction temperature, two phases were formed automatically. The colourless product phase could be efficiently separated from the precipitate rhodium catalyst phase by centrifuge. The catalyst was reused for five times without obvious loss of rhodium and the catalytic activity. The rhodium leaching in product mixture was less than 0.03% of the total rhodium.


 Please wait while we load your content...
Please wait while we load your content...