Gelation and luminescence of lanthanide hydrogels formed with deuterium oxide†
Abstract
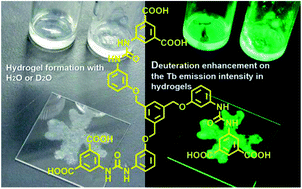
Gel formations and efficient lanthanide luminescence appeared in deuterium oxide (D2O) medium instead of light water (H2O), and their solvation possibilities by using luminescence lifetimes were discussed. The lanthanide ions in the hydrogel of 1 obtained by H2O (abbreviated as H2O-Ln1; Ln = Eu, Tb, and Gd) in our previous report act as the coupling part between neighbor molecules for the bundle structure. Here, D2O also acts as a medium to form the lanthanide-hydrogel of 1, and increases intensities of luminescence for Tb, because a soft crystalline state reducing resonance thermal relaxation is realized. The gel-formation and luminescence band positions of Ln1 in D2O corresponded to those in H2O. From the observation of luminescence lifetimes in H2O and D2O, the number of coordinating water molecules on Eu and Tb were estimated to be around 3 or 4 for both. The luminescence intensity of Eu1 did not increase even in D2O, due to a blue shift of the excited triplet state of 1, as compared to that in H2O.


 Please wait while we load your content...
Please wait while we load your content...