Absorption and thermodynamic properties of CO2 by amido-containing anion-functionalized ionic liquids†
Abstract
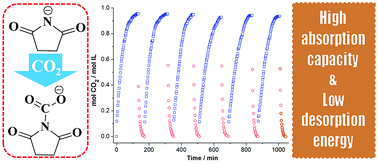
In this contribution, two kinds of amido-containing anion-functionalized ionic liquids (ILs) were designed and synthesized, where the anions of these ILs were selected from deprotonated succinimide (H-Suc) and o-phthalimide (Ph-Suc). Then, these functionalized ILs were used to capture CO2. Towards to this end, solubility of CO2 in the ILs was determined at different temperatures and different CO2 partial pressures. Based on these data, chemical equilibrium constants of CO2 with the ILs were derived at different temperatures from the “deactivated IL” model. The other thermodynamic properties such as reaction Gibbs energy, reaction enthalpy, and reaction entropy in the absorption were also calculated from the corresponding equilibrium constant data at different temperatures. It was shown that these anion-functionalized ILs exhibited high CO2 solubility (up to 0.95 mol CO2 mol−1 IL) and low energy desorption, and enthalpy change was the main driving force for CO2 capture by using such ILs as absorbents. In addition, the interactions of CO2 with the ILs were also investigated by 1H NMR, 13C NMR, and FT-IR spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...