Synthesis and characterization of methylammonium phosphates as crystalline approximants for anhydrous, low melting phosphate glasses†
Abstract
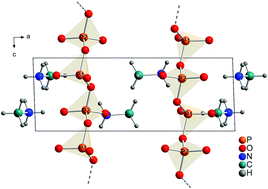
Low-melting methylammonium phosphate glasses are synthesized from crystalline starting agents. To this end crystalline tris(methylammonium) cyclotriphosphate [CH3NH3]3P3O9, was synthesized by a novel and simple synthesis route from P4O10 and N-methylformamide. It, undergoes an irreversible phase transition to methylammonium catena-polyphosphate [CH3NH3]PO3. The crystal structure of the catena-polyphosphate was solved and refined from X-ray powder diffraction data by the Rietveld method using constraints obtained by solid-state 31P and 1H NMR spectroscopy. This compound crystallizes in a triclinic space group with a = 13.2236(9), b = 7.8924(6), c = 4.6553(2) Å, α = 91.068(4), β = 87.840(5) and γ = 106.550(3)°. Quantum chemical calculations confirm that the obtained structure lies at an energetic minimum. Finally the reaction of tris(methylammonium) cyclotriphosphate and P4O10 into methylammonium phosphate glass is presented. The synthesized, water-free phosphate glass shows a very low glass transition temperature Tg of 33 °C, which was verified by dynamic scanning calorimetry and NMR. The chain-like crystal structure of the high-temperature methylammoniumphosphate [CH3NH3]PO3 serves as an approximation for the short-range order of the glass.


 Please wait while we load your content...
Please wait while we load your content...