Non-photochemical catalytic hydrolysis of methyl parathion using core–shell Ag@TiO2 nanoparticles†
Abstract
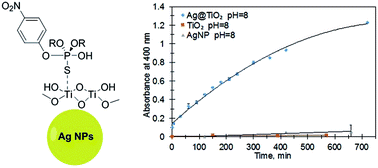
Highly water-dispersible core–shell Ag@TiO2 nanoparticles were prepared and shown to be catalytically active for the rapid degradation of the organothiophosphate pesticide methyl parathion (MeP). Formation of the hydrolysis product, p-nitrophenolate was monitored at pH 7.5 and 8.0, using UV-Vis spectroscopy. 31P NMR spectroscopy confirmed that hydrolysis is the predominant pathway for substrate breakdown under non-photocatalytic conditions. We have demonstrated that the unique combination of TiO2 with silver nanoparticles is required for catalytic hydrolysis with good recyclability. This work represents the first example of MeP degradation using TiO2 doped with AgNPs under mild and ambient conditions. Analysis of catalytic data and a proposed dark mechanism for MeP hydrolysis using core–shell Ag@TiO2 nanoparticles are described.


 Please wait while we load your content...
Please wait while we load your content...