Racemic vs. enantiopure inert Ti(iv) complex of a single diaminotetrakis(phenolato) ligand in anticancer activity toward human drug-sensitive and -resistant cancer cell lines†
Abstract
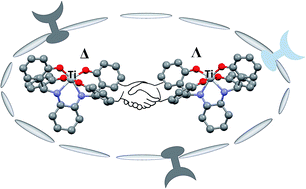
A tetrakis(phenolato) Ti(IV) complex was synthesized in racemic and optically pure form, exhibiting high hydrolytic stability, and similar cytotoxicity for all stereochemical forms on HT-29 and A2780 cancer cells. Higher activity of the racemate on drug-resistant A2780cp and A2780adr lines implies a beneficial activity of both enantiomers rendering enantiomeric resolution unnecessary.


 Please wait while we load your content...
Please wait while we load your content...