Removal of arsenic(v) from aqueous solutions using sulfur-doped Fe3O4 nanoparticles†
Abstract
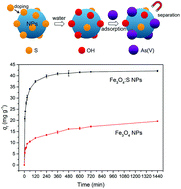
In this study, magnetic sulfur-doped Fe3O4 nanoparticles (Fe3O4:S NPs) were applied as adsorbents for the removal of As(V). Fe3O4:S NPs were fabricated by a two-step route, which included low-temperature mixing and high-temperature sintering. The as-prepared Fe3O4:S NPs could effectively remove As(V) under a wide pH range of 2–10 and presented a high As(V) adsorption capacity of 58.38 mg g−1, which was much better than undoped Fe3O4 nanoparticles (20.24 mg g−1). Adsorption experiments exhibited a pseudo-second-order model of adsorption kinetics and a Langmuir isotherm model of adsorption isotherms. Additionally, the coexisting ions such as NO3−, SO42−, and CO32− had no significant effect on As(V) adsorption and the adsorbent worked well in actual smelting wastewater. XPS and FTIR spectra of Fe3O4:S NPs before and after As(V) adsorption showed that Fe–OH groups played a significant role in the adsorption mechanisms. Moreover, the magnetic Fe3O4:S NPs adsorbents after adsorption could be rapidly separated from wastewater with an external magnetic field. Therefore, Fe3O4:S NPs could be an ideal candidate for the removal of As(V) from water.


 Please wait while we load your content...
Please wait while we load your content...