Magnetic solid-phase extraction using a mixture of two types of nanoparticles followed by gas chromatography-mass spectrometry for the determination of six phthalic acid esters in various water samples†
Abstract
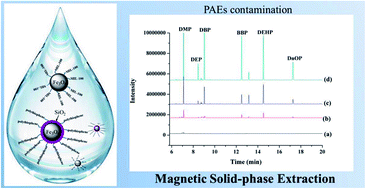
Two types of magnetic microspheres (Fe3O4@MIL-100 and Fe3O4@SiO2@polythiophene) were prepared and characterized as mixed sorbents for magnetic solid-phase extraction (MSPE) of six phthalic acid esters (PAEs), including dimethyl phthalate (DMP), diethyl phthalate (DEP), di-n-butyl phthalate (DBP), benzyl butyl phthalate (BBP), di-2-ethylhexyl phthalate (DEHP), and di-n-octyl phthalate (DnOP) from water samples prior to gas chromatography-mass spectrometry (GC-MS) analysis. The synthetic magnetic nanocomposites exhibited good repeatability and chemical stability, and improved extraction efficiency for the tested PAEs. The mixture of the two types of nanoparticles substantially improved the extraction efficiency of both DMP and DEP. The key parameters affecting the extraction efficiency, such as the type and the amount of sorbent, eluent (desorption solvent), adsorption and desorption time, pH of sample solution, and sample volume, were investigated and optimized, respectively. Under optimized conditions, the developed method showed satisfactory linearity in the range of 5–5000 μg L−1 with coefficients of determination (R2) > 0.9935. The method detection limits (MDLs) and limits of quantitation (LOQs) were between 0.35–0.91 μg L−1 and 1.1–2.9 μg L−1, respectively. At three fortification levels (1.0, 10.0, and 50.0 μg L−1), the mean recoveries ranged from 76.9–109.1% with favorable relative standard deviations (RSDs) < 9%. The feasibility of the method was evaluated by analysis of water samples from various sources (tap, drinking, and mineral water). The results show that the developed method is suitable for determination of trace level PAEs in water samples.


 Please wait while we load your content...
Please wait while we load your content...