Metal free oxidation of vinamidine derivatives: a simple synthesis of α-keto-β-diimine ligands†
Abstract
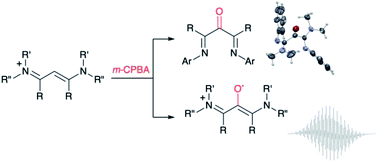
Oxidation of vinamidinium salts with meta-chloroperbenzoic acid is the key synthetic step towards new persistent 1,3-di(amino)oxyallyl radical cations. When applied to parent vinamidines, this protocol allows for a simple straightforward synthesis of α-keto-β-diimine ligands, for which no convenient synthesis was previously available.


 Please wait while we load your content...
Please wait while we load your content...