Synthesis of novel 1,2,4-thiadiazinane 1,1-dioxides via three component SuFEx type reaction†
Abstract
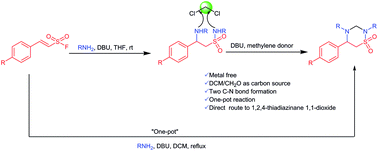
Herein, we report the preparation of 1,2,4-thiadiazinane 1,1-dioxides from reaction of β-aminoethane sulfonamides with dichloromethane, dibromomethane and formaldehyde as methylene donors. The β-aminoethane sulfonamides were obtained through sequential Michael addition of amines to α,β-unsaturated ethenesulfonyl fluorides followed by further DBU mediated sulfur(VI) fluoride exchange (SuFEx) reaction with amines at the S–F bond.


 Please wait while we load your content...
Please wait while we load your content...