Metal-free hypervalent iodine/TEMPO mediated oxidation of amines and mechanistic insight into the reaction pathways†
Abstract
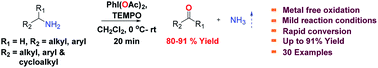
A highly efficient metal free approach for the oxidation of primary and secondary amines to their corresponding aldehydes and ketones using PhI(OAc)2 in combination with a catalytic amount of TEMPO as an oxidizing agent is described. This protocol is rapid and provides diverse products under milder reaction conditions in excellent yields. In addition, the mechanistic study is well demonstrated by spectroscopic methods.
- This article is part of the themed collection: How does it work? – a collection on mechanistic studies


 Please wait while we load your content...
Please wait while we load your content...