An efficient and atom-economical route to N-aryl amino alcohols from primary amines†
Abstract
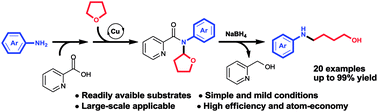
In this paper we reported a novel method for generation of N-aryl amino alcohols from N,N-disubstituted picolinamides through reduction/ring-opening reaction with NaBH4. The N,N-disubstituted picolinamides can be easily obtained from primary amines after convenient condensation with picolinic acid and coupling with cyclic ethers. The whole route proceeded under simple and mild conditions with high efficiency. Picolinic acid can be recovered in the form of piconol after reaction. It indicated an efficient and atom-economical route for the preparation of N-aryl amino alcohols from primary amines.


 Please wait while we load your content...
Please wait while we load your content...