Synthesis and conductive performance of polyoxometalate acid salt gel electrolytes
Abstract
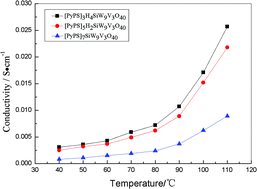
Two vanadium-substituted polyoxometalate acid salt gel electrolytes, [PyPS]3H4SiW9V3O40 and [PyPS]5H2SiW9V3O40, have been synthesized using a 1-(3-sulfonic group) propylpyridine (PyPS) and a Keggin vanadium-substituted heteropoly acid H7SiW9V3O40 through an ionic self-assembly method, and adjusting the ratio of cation and anion. A substitution effect of the acid salt gel electrolytes has been investigated. Interestingly, when protons of the polyoxometalate acid salt gel electrolytes are substituted, both the conductivity and the phase transformation temperature increase. The fastest conductivity of these gel electrolytes was as high as 2.57 × 10−2 S cm−1 at 110 °C.


 Please wait while we load your content...
Please wait while we load your content...