Facile fabrication of hierarchical film composed of Co(OH)2@Carbon nanotube core/sheath nanocables and its capacitive performance†
Abstract
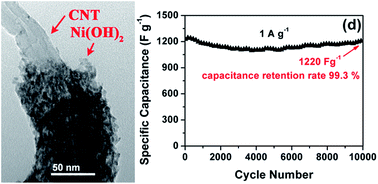
A hierarchical film composed of Co(OH)2@carbon nanotube (CNT) core/sheath nanocables (CCNF) was generated via a simple and rapid electrophoretic deposition method. It is found that the Co(OH)2 sheath was uniformly anchored on the surface of conductive CNT core. The Co(OH)2 sheath, with a thickness of ∼20 nm, was composed of numerous very tiny nanoparticles. Such a unique nanostructure endows the CCNF with a high surface area of 126 m2 g−1 and a hierarchical porosity, resulting in a large accessible surface area for redox activity. As expected, the CCNF exhibits high specific capacitance and excellent rate performance. Its specific capacitance reached 1215 F g−1 under a low current density of 1 A g−1 and was maintained at 832 F g−1 when the current density was increased 20 times to 20 A g−1. A high capacitance retention of 99.3% was achieved after 10 000 cycles at 1 A g−1. Such intriguing capacitive behavior is attributed to the synergistic effect of the CNT core and the Co(OH)2 sheath.


 Please wait while we load your content...
Please wait while we load your content...