The adsorption and aggregation properties of dendritic cationic tetrameric surfactants†
Abstract
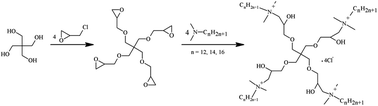
A series of dendritic cationic tetrameric surfactants (4CntetraQ, n = 12, 14, 16) were synthesized with raw materials that are commercially available. The adsorption and aggregation properties of the synthesized surfactants were studied using the Wilhelmy plate method, electrical conductivity, fluorescence spectra and dynamic light scattering techniques, and surface chemical parameters and thermodynamics parameters were obtained, respectively. These surfactants have higher surface activities than those of the corresponding monomeric and dimeric surfactants and are more prone to self-assembling in aqueous solution than adsorption at the surface. The thermodynamics parameters reflect that both adsorption and micellization processes of 4CntetraQ are spontaneous, and that the micellization process is entropy-driven. Both adsorption and micellization of 4CntetraQ have a tendency to occur with increasing alkyl chain length or temperature. DLS measurements show that there exist large aggregates at the concentration of 10 times the CMC.


 Please wait while we load your content...
Please wait while we load your content...