Water adsorbancy of high surface area layered double hydroxides (AMO-LDHs)†
Abstract
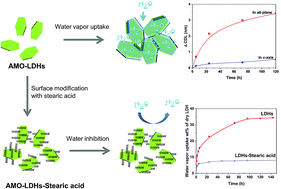
Understanding the water adsorbancy of highly dispersed, high surface area layered double hydroxide (LDH) is of great importance as it directly relates to their hydrophobicity and subsequent use as additives in LDH-polymer nanocomposites. In this study, we have investigated the water vapour uptake response of highly dispersed, high surface area aqueous miscible organic-LDHs (AMO-LDHs) in two relative humidity atmospheres (RH99 and RH60) at 20 °C. We observed that AMO-Mg3Al–CO3 and AMO-Zn2MgAl–CO3 exhibited very high water vapour uptake in an RH99 atmosphere at 20 °C (56 wt% and 20 wt% for Mg3Al–CO3 and Zn2MgAl–CO3 LDH respectively after 120 h). The crystallinity in both ab-plane and c-axis of the LDHs increased with increasing exposure uptake. The water vapour adsorption capacity of the AMO-LDHs can be dramatically reduced by treatment with stearic acid.


 Please wait while we load your content...
Please wait while we load your content...