Anion influences on reactivity and NMR spectroscopic features of NHC precursors†
Abstract
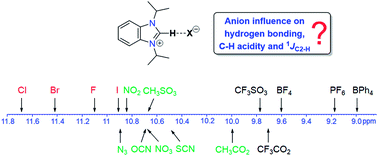
A series of 16 benzimidazolium salts of the type iPr2-bimyH+X− with various anions X were synthesized and characterized by various spectroscopic and spectrometric methods. Significant anion and solvent effects on the chemical shifts of the C2–H protons were found, which allows for a ranking of the anions in terms of their hydrogen-bond acceptor properties. Stronger acceptors could increase the acidity of their respective salts leading to a faster H/D exchange. Similar but less pronounced anion influences were detected for the 13CC2 NMR resonances, while 1JC2–H coupling constants appear to be anion and solvent independent.


 Please wait while we load your content...
Please wait while we load your content...