Synthesis of an oxygen-linked germinal frustrated Lewis pair and its application in small molecule activation†
Abstract
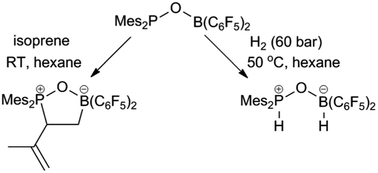
Reaction of Mes2P(O)Li with (C6F5)2BCl gave access to an oxygen-linked germinal intramolecular frustrated Lewis pair Mes2P(O)B(C6F5)2 (1). Compound 1 is stable at room temperature and only decomposes when heated to 90 °C. NMR analysis and theoretical analysis revealed the frustrated nature between the boron and phosphorus centers. Compound 1 shows typical frustrated Lewis pair reactivity when treated with dihydrogen, carbon dioxide, alkyne and alkene. The reaction of 1 with isoprene resulted in selective formation of 3,4-phosphoryl/boryl addition product 8.


 Please wait while we load your content...
Please wait while we load your content...