Efficient bioconversion of furfural to furfuryl alcohol by Bacillus coagulans NL01†
Abstract
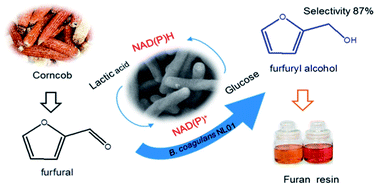
Bio-catalysis is an attractive alternative to replace chemical methods due to its high selectivity and mild reaction conditions. Furfural is an important bio-based platform compound generated from biomass. Herein, the bio-catalytic reduction of furfural (FAL) to furfuryl alcohol (FOL) was performed by using a furfural tolerant strain, Bacillus coagulans NL01. An efficient co-substrate was explored and a high conversion and selectivity of FAL to FOL was reported over this bio-catalytic system using glucose as co-substrate. As the bioconversion occurred over 42 mM FAL, 20 g L−1 glucose and 9 mg mL−1 at 50 °C, a high conversion and selectivity was obtained by 3 h reaction. This transformation rate of FAL was the highest compared with other reactions. Furthermore, about 98 mM FOL was produced from FAL within 24 h by a fed-batch strategy with a conversion of 92% and selectivity of 96%. These results indicate that this bio-catalytic reduction of FAL has high potential for application to upgrading of FAL and B. coagulans NL01 is a promising biocatalyst for the synthesis of FOL. In addition, this bio-catalytic reduction shows a high potential application for catalytic upgrading of FAL from biomass.


 Please wait while we load your content...
Please wait while we load your content...