High electrochemical performance of nanocrystallized carbon-coated LiFePO4 modified by tris(pentafluorophenyl) borane as a cathode material for lithium-ion batteries
Abstract
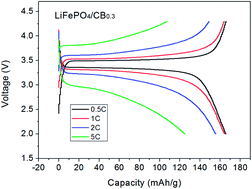
Tris(pentafluorophenyl) borane (C18BF15) was first adopted as a boron source, which clearly demonstrated its modification effects. XPS and EDX mapping proved that boron can be successfully doped into a carbon layer. The high number of defects in the carbon induced by boron was demonstrated via Raman spectroscopy and thus, the electric conductivity of LiFePO4 was greatly enhanced. The boron-doped composite possessed a higher specific discharge capacity and rate capability than the undoped sample. For instance, the reversible specific capacity for the boron-doped cathode reached 165.8 mA h g−1 at 0.5C, which was almost close to its theoretical capacity (166 mA h g−1). Even at a high rate of 5C, it still possessed a high specific capacity of 124.8 mA h g−1. This provides for the possibility that boron-doped carbon-coated LiFePO4 cathodes may deliver high energy and power density for rechargeable lithium-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...