New insights into the effect of morphology on catalytic properties of MnOx–CeO2 mixed oxides for chlorobenzene degradation
Abstract
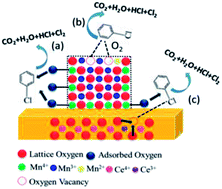
We synthesized four CeO2–MnOx mixed oxides with different morphologies using simple hydrothermal methods. The catalytic activity for chlorobenzene (CB) degradation decreases in the following order: rod-CeO2–MnOx > plate-CeO2–MnOx > polyhedra-CeO2–MnOx > cube-CeO2–MnOx. CeO2 and MnOx in the mixed oxides are highly dispersed and two new phases of both todorokite (S.G.: P2/m:b) and vernadite (S.G.: I4/m) with a special tunnel-like structure are found. Both rod-CeO2–MnOx and plate-CeO2–MnOx exhibit increased lattice microstrains generated from lattice distortion and defects; further, there are more oxygen vacancies and more MnOx (Mn4+ and Mn2+) species on the surface, particularly when compared to cube-CeO2–MnOx. Therefore, this promotes deeper oxidation activity for CB. Moreover, the strong interaction between CeO2 and MnOx also promotes the redox ability of CeO2–MnOx mixed oxides, while their oxygen storage capacity (OSC) properties are not only intrinsic to their structures but also limited to their surfaces and by their particle sizes.


 Please wait while we load your content...
Please wait while we load your content...