Ag2O nanoparticle-catalyzed substrate-controlled regioselectivities: direct access to 3-ylidenephthalides and isocoumarins†
Abstract
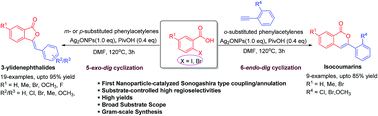
Herein, we disclose the first example of an efficient, silver oxide nanoparticle-catalyzed, direct regioselective synthesis of 3-ylidenephthalides 11–16 and isocoumarins 17–20 via sonogashira type coupling followed by substrate-controlled 5-exo-dig or 6-endo-dig cyclization reaction, respectively. This one pot coupling involves reaction of substituted 2-halobenzoic acid with meta/para-substituted and ortho-substituted terminal alkynes, which proceeded in a regioselective manner resulting in the formation of 3-ylidenephthalides or isocoumarins, respectively, in excellent yields (up to 95%) with complete Z-selectivity. This protocol features relatively broad substrate scope, mild conditions, operational simplicity, and is favourable with aromatic/alicyclic terminal alkynes. The competition experiments and gram-scale synthesis further highlight the importance and versatility of the methodology. The proposed mechanistic pathways illustrate that the regioselectivity is substantially being controlled by the substituent(s) present on the acetylenic phenyl ring.


 Please wait while we load your content...
Please wait while we load your content...