C–H activation-annulation on the N-heterocyclic carbene platform
Abstract
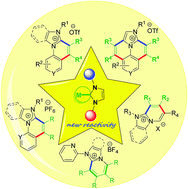
Ring-fused cationic N-heterocycles are an important class of organic compounds recognized to be of significant interest in diverse research areas including bioactivity, materials chemistry, supramolecular chemistry, etc. Toward the synthesis of such molecules, recently unique chemistry has been explored utilizing a novel conjugative action of NHC ligands as a functionalizable directing group in rhodium(III)-catalyzed aromatic/heteroaromatic/non-aromatic C–H activation and subsequent annulation of various imidazolium salts with internal alkynes. This review highlights the initial development and underscores the potential of this chemistry.
- This article is part of the themed collection: Chemical Frontiers Goa


 Please wait while we load your content...
Please wait while we load your content...