Copper-catalyzed domino sequences: a new route to pyrido-fused quinazolinones from 2′-haloacetophenones and 2-aminopyridines†
Abstract
A new pathway to access pyrido-fused quinazolinones via a Cu(OAc)2-catalyzed domino sequential transformation between 2′-haloacetophenones and 2-aminopyridines was demonstrated. The solvent and base exhibited a remarkable effect on the transformation, in which the combination of DMSO and NaOAc emerged as the best system. Cu(OAc)2·H2O was more active towards the reaction than numerous other catalysts. This methodology is new and would be complementary to previous protocols for the synthesis of pyrido-fused quinazolinones.
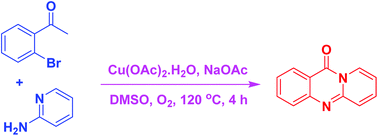


 Please wait while we load your content...
Please wait while we load your content...