Synergistic effect of temperature and background counterions on ion-exchange equilibria
Abstract
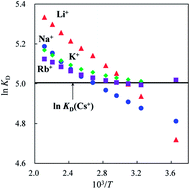
The effects of temperature and background counterions on ion-exchange selectivity for alkali metal ions and tetraalkylammonium ions on strongly acidic cation-exchange resins have been investigated using superheated water ion-exchange chromatography (SW-IEC). We have found out that alkali metal ions show reversal in the order of the distribution coefficient (KD), from Li+ < Na+ < K+ < Rb+ in water at ordinary temperature to Rb+ < K+ < Na+ < Li+ in superheated water, when a relatively large cation such as cesium ion is used as the background counterion. The effect of counterion on the ion-exchange selectivity is enhanced with the ion-exchange resins of higher ion-exchange capacity and cross-linking degree. Tetraalkylammonium ions chosen as model ions for poorly hydrated ions also exhibit reversal in the order of KD at around 430 K in superheated water. However, the effect of the nature of alkali metal counterions on the change in KD values of tetraalkylammonium ions is rather small compared with the effect on the KD of alkali metal ions. These results are attributed to the change in local hydration structures of the ions in the ion-exchange resin due to dehydration of alkali metal ions enhanced by interionic contacts of the analyte ion with the coexisting counterion and lower hydration energy of the ions at elevated temperatures. Although it has been considered that temperature is not effective at changing the ion-exchange separation selectivity, significant selectivity changes can be achieved by SW-IEC.


 Please wait while we load your content...
Please wait while we load your content...