Direct speciation methods to quantify catalytically active species of AlCl3 in glucose isomerization†
Abstract
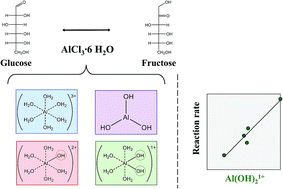
While homogeneous metal halides have been shown to catalyze glucose to fructose isomerization, direct experimental evidence in support of the catalytically active species remains elusive. Here, we integrate direct speciation methods with kinetics to provide strong evidence for the active species of AlCl3 in glucose–fructose isomerization in water. We investigate the effect of Lewis (AlCl3) and Brønsted (HCl) acids on aluminum hydrolysis and glucose conversion. We demonstrate the interplay between the acids using the Optimum Logic Inc. speciation model (OLI software). We measure aqueous aluminum species and protons through in situ and ex situ 27Al quantitative nuclear magnetic resonance (qNMR) and pH measurements, respectively, and quantify aluminum nanoparticles through a combination of inductively coupled plasma-mass spectrometry (ICP-MS), dynamic light scattering (DLS), and ultrafiltration. Direct speciation measurements correlated with the glucose isomerization rate indicate that the hydrolyzed Al(III) complex [Al(H2O)4(OH)2]1+ is the active species in glucose isomerization.


 Please wait while we load your content...
Please wait while we load your content...