Imido-substituted triazines as dehydrative condensing reagents for the chemoselective formation of amides in the presence of free hydroxy groups†
Abstract
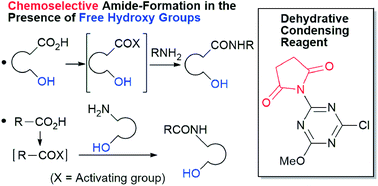
In this paper, we discuss the synthesis of imido-substituted chlorotriazines and demonstrate their use in dehydrative condensation reactions. Chemoselective amide-forming reactions of amino alcohols using succinimido-substituted chlorotriazine (2A) proceeded smoothly. Occasionally, nonselectivity was problematic during the synthesis of hydroxy-substituted amides. Moreover, it was noteworthy that this method was applicable to hydroxy-substituted carboxylic acids that could have formed a lactone or an ester during the carboxylic acid activation step. The imido-substituted chlorotriazine (2A) was superior to the amido-substituted chlorotriazine and 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) in terms of reaction rates and yields.


 Please wait while we load your content...
Please wait while we load your content...