A convenient approach to difluoromethylated all-carbon quaternary centers via Ni(ii)-catalyzed enantioselective Michael addition†
Abstract
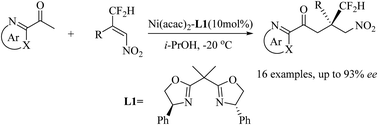
A Ni(II)-catalyzed enantioselective Michael addition of 2-acetyl azarenes with β-difluoromethyl substituted nitroalkenes was successfully realized, which afforded chiral CF2H-containing compounds in good enantioselectivities (up to 93% ee). This protocol provides a new convenient approach to all-carbon quaternary stereogenic centers featuring a CF2H group.


 Please wait while we load your content...
Please wait while we load your content...