Off-stoichiometric Na3−3xV2+x(PO4)3/C nanocomposites as cathode materials for high-performance sodium-ion batteries prepared by high-energy ball milling†
Abstract
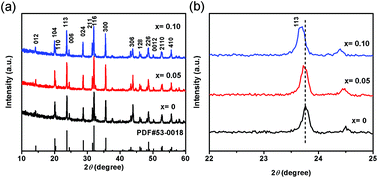
Na3V2(PO4)3 (NVP) is regarded as a promising cathode material for sustainable energy storage applications. Here we present an efficient method to synthesize off-stoichiometric Na3−3xV2+x(PO4)3/C (x = 0–0.10) nanocomposites with excellent high-rate and long-life performance for sodium-ion batteries by high-energy ball milling. It is found that Na3−3xV2+x(PO4)3/C nanocomposites with x = 0.05 (NVP-0.05) exhibit the most excellent performance. When cycled at a rate of 1C in the range of 2.3–3.9 V, the initial discharge capacity of NVP-0.05 is 112.4 mA h g−1, which is about 96% of its theoretical value (117.6 mA h g−1). Even at 20C, it still delivers a discharge capacity of 92.3 mA h g−1 (79% of the theoretical capacity). The specific capacity of NVP-0.05 is as high as 100.7 mA h g−1 after 500 cycles at 5C, which maintains 95% of its initial value (106 mA h g−1). The significantly improved electrochemical performance of NVP-0.05 is attributed to the decrease of internal resistance and increase of the Na+ ion diffusion coefficient.


 Please wait while we load your content...
Please wait while we load your content...