Non-isothermal TGA study on the combustion reaction kinetics and mechanism of low-rank coal char
Abstract
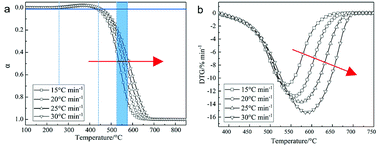
In this paper, the combustion reaction kinetics of pyrolysis char of low-rank coal is studied by thermal analysis technology. For the combustion process of the char at different heating rates, the reaction kinetic parameters were calculated by three common mode-free methods (FWO method, KAS method and Starink method); the reaction model was determined by Malek method and Popescu method. Research shows that activation energy Eα of char combustion calculated by the three methods was 110.66–70.31 kJ mol−1, 104.35–59.60 kJ mol−1 and 104.34–59.99 kJ mol−1, respectively, and the activation energy decreased with increasing conversion rates. There is a compensation effect between the activation energy and pre-exponential factor of char combustion. Results of the kinetic analysis by Malek method and Popescu method both indicated that the Avrami–Erofeev equation (n = 3/2) (f(α) = 3/2(1 − α)[−ln(1 − α)]1/3) controlled by nucleation and nuclei growth models is the most probable reaction model of char combustion.


 Please wait while we load your content...
Please wait while we load your content...