Oxygen release from metal oxide for repeated hydrogen regeneration by proton irradiation with polyvinylpyrrolidone
Abstract
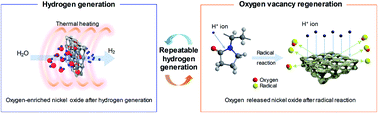
In this study, we investigated the reduction of a 3D microporous NiOx structure, used as a metal oxide catalyst, by proton irradiation with polyvinylpyrrolidone (PVP) for hydrogen regeneration. In general, the reduction process for hydrogen regeneration requires high temperatures (1000–4000 °C) to release saturated oxygen from the metal oxide catalyst. Proton irradiation with PVP could regenerate abundant oxygen vacancies by releasing the oxygen attached to NiOx at room temperature. The 3D microporous NiOx structure provided the maximum hydrogen generation rate of ∼4.2 μmol min−1 g−1 with the total amount of generated hydrogen being ∼460 μmol g−1 even in the repetitive thermochemical cycle; these results are similar to the initial hydrogen generation data. Therefore, continuous regeneration of hydrogen from the oxygen-reduced 3D microporous NiOx structure was possible. It is expected that the high thermal energy, which is the major problem associated with hydrogen regeneration through the conventional heat treatment method, would be resolved in future using such a method.


 Please wait while we load your content...
Please wait while we load your content...