A julolidine-fused anthracene derivative: synthesis, photophysical properties, and oxidative dimerization†
Abstract
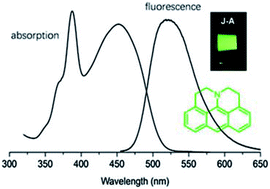
We describe the synthesis and characterization of a julolidine-fused anthracene derivative J-A, which exhibits a maximum absorption of 450 nm and a maximum emission of 518 nm. The fluorescent quantum yield was determined to be 0.55 in toluene. J-A dimerizes in solution via oxidative coupling. Structure of the dimer was characterized using single crystal X-ray diffraction.


 Please wait while we load your content...
Please wait while we load your content...