Ag–K/MnO2 nanorods as highly efficient catalysts for formaldehyde oxidation at low temperature
Abstract
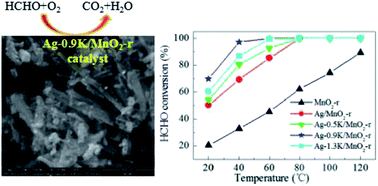
A series of Ag–K/MnO2 nanorods with various molar ratios of K/Ag were synthesized by a conventional wetness incipient impregnation method. The as-prepared catalysts were used for the catalytic oxidation of HCHO. The Ag–K/MnO2 nanorods with an optimal K/Ag molar ratio of 0.9 demonstrated excellent HCHO conversion efficiency of 100% at a low temperature of 60 °C. The structures of the samples were investigated by BET, TEM, SEM, XRD, H2-TPR, O2-TPD and XPS. The results showed that Ag–0.9K/MnO2-r exhibited more facile reducibility and greatly abundant surface active oxygen species, endowing it with the best catalytic activity of the studied catalysts. This work provides new insights into the development of low-cost and highly efficient catalysts for the removal of HCHO.


 Please wait while we load your content...
Please wait while we load your content...